Measuring Osmolarity in Science and Industry: Applications, Methods, and the KNAUER Osmometer K-7400S
Osmolarity – the concentration of dissolved particles in a solution – is a fundamental parameter in chemistry and biology. It indicates how many osmotically active substances are present and thus how a solution will interact with cells or membranes through osmosis . In scientific research and industrial quality control, osmolarity (often expressed in milliosmoles per liter or kilogram) plays a critical role in ensuring solutions have appropriate concentrations for their intended use. From making sure intravenous drips won’t damage blood cells to verifying sports drinks are truly “isotonic,” accurate osmolarity measurement underpins product safety and efficacy. This article explores what osmolarity is, why it matters across various industries, how it is measured using freezing point depression, and highlights the features of the KNAUER Osmometer K-7400S – a state-of-the-art instrument for fast and precise osmolarity determination.
Osmolarity and Its Significance in Scientific Research
In scientific terms, osmolarity (and the closely related measure *osmolality*) quantifies the total number of solute particles in a solution. Unlike concentration in molarity, osmolarity counts *all* particles (ions, molecules) that affect osmotic pressure. This makes it a key factor in colligative properties of solutions, which include osmotic pressure, boiling point elevation, and freezing point depression . When a solute is dissolved in a solvent, the presence of these particles lowers the solvent’s freezing point and raises its boiling point proportionally to the particle concentration . Thus, by measuring osmolarity, scientists gain insight into a solution’s behavior in processes like osmosis and phase changes. Maintaining proper osmolarity is crucial in many research applications – for example, cell culture media must be isotonic (having osmolarity similar to cells) to prevent cell damage, and biochemical assays often require specific osmotic conditions for accurate results. In clinical research, body fluids such as blood or urine are analyzed for osmolality to assess patient hydration and kidney function, underlining osmometry’s importance in physiology.
Osmometry – the measurement of osmotic concentration – is widely used as a quality control and analytical tool across numerous fields . Precise osmolarity measurements ensure that formulated solutions behave as expected: an error in osmolarity could mean an eye drop causes irritation, or a “hydrating” drink actually dehydrates. The significance of osmolarity spans from fundamental research (where it affects experimental reproducibility) to industrial production (where it impacts product safety and regulatory compliance). In the following sections, we will look at key industries that rely on osmolarity measurements and why this parameter is so vital in each context.
Osmolarity in the Pharmaceutical Industry
Accurate osmolarity measurement is especially critical in the pharmaceutical industry, where it directly affects product safety and efficacy. Many pharmaceutical formulations – such as intravenous infusion solutions, injectables, ophthalmic solutions (eye drops), and nasal sprays – must be isotonic with body fluids to avoid adverse reactions. For example, an IV infusion that is too concentrated or too dilute can cause red blood cells to shrink or burst, or lead to patient discomfort and electrolyte imbalances. To prevent this, pharma quality control labs routinely test the osmolality of solutions to ensure they match the body’s natural osmotic concentration (around 290 mOsm/kg for blood plasma) . Eye drops and contact lens solutions provide another clear example: if their osmolarity deviates too far from tears, they can cause stinging or dry eyes. Thus, pharmacopoeias and regulatory bodies often require osmolarity (or osmolality) testing for these products as part of release specifications.
Glass ampoulles as used for anesthetics, anti-inflammatory drugs and painkillers.
Photo: Fotolia
Precise osmometry is used in pharmaceutical manufacturing and quality control laboratories to verify formulation consistency . Freezing point depression osmometers are common instruments in pharma QC labs, valued for their accuracy and reliability. They help confirm that each batch of, say, a saline drip or dialysis fluid has the correct concentration of solutes. This not only ensures patient safety but also consistency in therapeutic effect. Indeed, freezing point osmometers are frequently employed in clinical chemistry and drug manufacturing settings . For instance, osmometry is used during the development of lens care solutions and eye drops to achieve the ideal osmotic strength . By measuring osmolarity, scientists can adjust formulations to be hypotonic, isotonic, or hypertonic as needed for the product’s intended use. In summary, pharmaceutical companies rely on osmolarity measurements to guarantee that injectable and topical solutions are safe, comfortable, and effective for patients, maintaining strict compliance with pharmacopeial standards for osmotic concentration.
The Critical Role of Osmolarity in Lipid Nanoparticle (LNP) Quality Control for Vaccines
In recent years, lipid nanoparticle (LNP) technology has emerged as a transformative method in vaccine development, notably enabling the rapid deployment of effective mRNA vaccines. A crucial aspect of ensuring safety and efficacy in these advanced pharmaceutical products is rigorous quality control—including accurate osmolarity measurements.
Osmolarity plays a pivotal role in LNP formulations because it directly influences the stability, bioavailability, and tolerability of vaccines. LNP-encapsulated vaccines, such as those used in mRNA COVID-19 vaccines, must closely match physiological osmotic conditions to prevent adverse reactions upon administration. Deviations in osmolarity can lead to instability in LNP structure, reducing vaccine potency and potentially causing discomfort or cellular stress upon injection.
Freezing point osmometry, such as that performed by KNAUER's K-7400S Semi-Micro Osmometer, is an indispensable analytical technique in this context. It ensures that vaccine formulations maintain optimal osmolarity levels, providing reliable measurements critical for batch consistency and regulatory compliance. Precise osmolarity control guarantees that LNP vaccines remain safe, stable, and effective from manufacturing through administration.
Thus, osmolarity is not merely a technical specification but a cornerstone of vaccine quality assurance, supporting both patient safety and therapeutic effectiveness in modern vaccine technology.
Osmolarity in the Food & Beverage Industry
Sports and nutritional drinks are often marketed as “isotonic,” meaning their osmolarity is comparable to that of human blood for optimal hydration. Osmolarity testing is crucial in the food and beverage industry to validate such claims and to inform product development. An isotonic beverage (typically 270–330 mOsm/kg) promotes efficient water and electrolyte absorption, making it ideal for athletes and anyone needing rapid rehydration . Regulatory definitions reinforce this: for example, the European Food Safety Authority (EFSA) stipulates that drinks labeled “isotonic” must have an osmolality between 270 and 330 mOsm/kg . Manufacturers use osmometry to ensure their sports drinks, electrolyte solutions, and rehydration fluids fall within this range and deliver the promised benefits. Osmometry is in fact the foremost tool to substantiate the osmolality of beverages according to such regulations . Without accurate osmolarity measurements, it would be extremely difficult to guarantee these products meet the required specifications.

Selection of sport drinks. Photo: pexels.com
Beyond sports drinks, osmolarity analysis is applied in broader food and beverage quality control. Formulators measure the osmolality of products like soft drinks, juices, dairy beverages, and even beer to understand their consistency and how they might interact with the human body. For instance, recent analyses have shown that some alcohol-free beers are nearly isotonic, suggesting they could hydrate as effectively as sports drinks . This kind of insight, gained through freezing point osmometry, can drive innovation in functional beverages. During production, maintaining target osmolarity ensures each batch has the same taste and mouthfeel, since solute concentration can affect sweetness and texture. The food industry also uses osmolarity tests in process development – for example, controlling the concentration of syrups or fermentation broths. In all these cases, reliable osmolarity measurements help manufacturers achieve consistency, substantiate health claims, and comply with food safety standards.
Osmolarity in Environmental Analysis
Environmental scientists and water quality professionals also make use of osmolarity measurements to assess the concentration of dissolved substances in environmental samples. In water quality analysis, for example, the osmolality of a water sample reflects its total concentration of salts and soluble compounds – essentially, it’s an indicator of salinity or pollutant load. Freshwater has a low osmolarity, whereas brackish or polluted water will have elevated levels. By measuring osmolarity, researchers can monitor changes in rivers, lakes, or groundwater that might signal contamination (such as runoff carrying fertilizers or road salts). Osmometry thus aids in environmental testing by providing a quick measure of total dissolved solids in various water sources, supporting ecosystem health evaluations. High osmolality in a lake could affect aquatic life by causing osmotic stress to fish and amphibians, so tracking this parameter helps in managing habitats and assessing compliance with environmental regulations for discharge waters.
Another application is in soil science and agriculture: the osmotic potential of soil water (which can be gauged via osmolality of soil extracts) influences plant growth and drought resistance. Environmental laboratories may use osmometers to determine the osmolality of soil solutions, giving insight into how easily plants can uptake water. Similarly, in climate and polar research, the salinity (and osmolality) of sea ice and brine is measured to understand freezing processes and marine ecosystem conditions. While not as common as in pharma or food labs, osmometry in environmental analysis provides valuable data on the chemical concentration of natural samples, complementing other measures like conductivity or specific ion analysis. These measurements can be important for regulatory compliance too – for instance, ensuring that wastewater being released will not drastically increase the osmotic pressure of the receiving waters, which could harm wildlife.
How Freezing Point Depression Osmometers Measure Osmolarity
To accurately measure osmolarity, modern labs often use the freezing point depression method implemented by osmometers. This technique is grounded in a simple physical principle: dissolving solutes in a liquid will lower its freezing point in direct proportion to the quantity of dissolved particles (a colligative property) . In practical terms, for an ideal dilute solution, adding 1 osmole of solute per kilogram of water depresses the freezing point by about 1.86 °C . Osmometers leverage this linear relationship by measuring how much a sample’s freezing point is depressed relative to pure water, and from that determining the osmolality (usually reported in mOsm/kg). The freezing point depression method is favored in laboratories because of its high accuracy and simplicity of execution. It directly senses a fundamental thermodynamic change (the freezing temperature), which correlates reliably with total solute concentration across a wide range of samples.
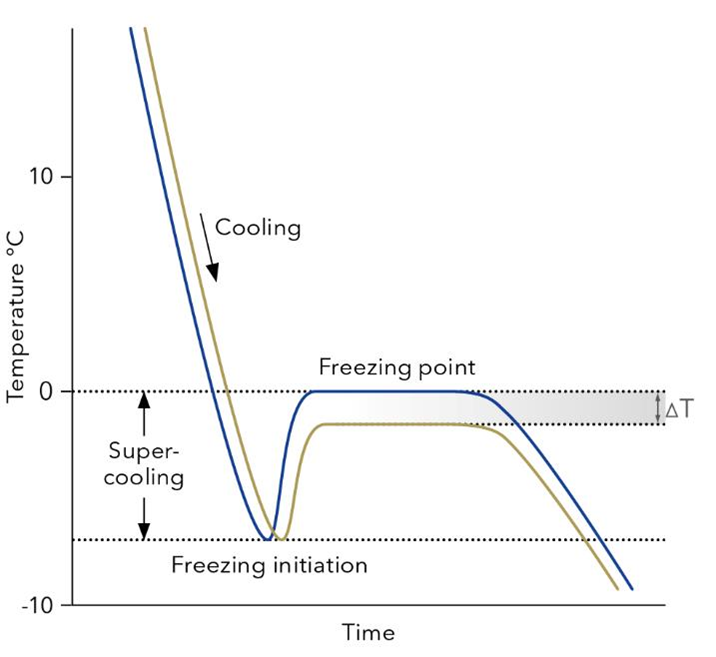
Fig. x Temperature curves of freezing point measurements. The sample is cooled down below 0 °C but still remains fluid (super cooling). At a predefined temperature the freezing process is initialized and after a short increase the temperature equilibrates at the true freezing point of the sample. While pure water freezes at 0 °C (blue), the presence of any solutes depresses a samples freezing point (yellow), which directly correlates with its osmolality.
In a freezing point osmometer, a small volume of the sample (typically 50–150 µL) is placed in a cooling chamber and super-cooled below 0 °C. At this point, the sample is still liquid but primed to freeze. The instrument then induces freezing – often by briskly stirring or “seeding” the sample – which causes ice crystals to form . As the sample begins to freeze, heat is released (the latent heat of fusion), momentarily raising the temperature until an equilibrium is reached. The osmometer detects this equilibrium plateau, which is the true freezing point of the sample . A high-precision thermistor monitors the temperature throughout, and because the device can resolve tiny temperature differences (on the order of 0.001 K) , even very small differences in osmolality between samples can be distinguished. The instrument is calibrated with solutions of known osmolality, so the measured freezing point can be converted directly to an osmotic concentration reading. In essence, the lower the freezing point, the more solute particles are in the sample, and the osmometer’s job is to translate that freezing point depression into an osmolarity value.
Freezing point osmometers are widely used for their precision and versatility. Unlike some other methods (such as vapor pressure osmometry), freezing point depression is not significantly affected by volatile solutes and can accommodate a variety of aqueous sample types. It is the predominant osmometry technique in clinical and industrial labs . Whether it’s a blood serum sample, a pharmaceutical injection, or a sports drink, the osmometer applies the same principle – freezing the sample and measuring its temperature change – to yield an accurate osmolality reading. The method is straightforward, fast (measurements typically take about 2 minutes per sample), and requires only a small sample volume, which makes it convenient and cost-effective for routine analysis.
The KNAUER Osmometer K-7400S: Features, Specifications, and Applications
KNAUER’s K-7400S Semi-Micro Osmometer is a high-performance freezing point osmometer designed to meet the rigorous demands of scientific and industrial osmometry. KNAUER, a pioneer in the field of osmometry for decades, has engineered the K-7400S to be reliable, user-friendly, and fast, building on proven technology to deliver reproducible results . This instrument determines the osmolality of aqueous solutions with ease and speed, requiring only a tiny sample and about two minutes per test. Such efficiency is invaluable in busy laboratories where many samples need analysis or where sample volume is limited (for instance, pediatric blood samples or precious research materials). The device uses a microprocessor-controlled Peltier cooler to precisely regulate temperature during the freezing process . This means no external cooling baths or ice needed – the solid-state cooling can rapidly super-cool and freeze the sample at the press of a button, with the process fully automated and monitored.
One of the standout advantages of the K-7400S is its semi-micro sample requirement: only 50–150 µL of sample is needed per measurement . Despite this small volume, the osmometer covers a broad osmolality range from 0 up to 2000 mOsmol/kg , enabling it to measure everything from extremely dilute solutions (like ultra-pure water or hypotonic fluids) to very concentrated ones (hypertonic solutions or concentrates) with high accuracy. The results are displayed as an integer mOsmol/kg value (with a resolution of 1 mOsmol/kg) on a clear LCD screen . For example, a reading might show “299 mOsmol” for an isotonic drink sample, as illustrated, indicating the beverage’s osmolality. The instrument’s precision is excellent – with a standard deviation ≤ 4 mOsm/kg in the low-med range and relative standard deviation ≤ 1% at higher concentrations – ensuring confidence in each measurement. Two-point calibration (typically using 0 and a mid-scale standard, e.g. 300 or 400 mOsm/kg) is simple to perform, and an optional three-point calibration can be done to span the range if needed . This flexibility makes it easy to maintain accuracy and comply with quality system requirements in regulated labs.

KNAUER Freezing Point Osmometer K-7400S in use
The K-7400S is equally notable for its robust and intelligent design. It is a standalone compact bench-top unit (about 16 cm × 18 cm footprint) with an integrated control panel, allowing all functions to be accessed via its keypad and menu – from starting a measurement to configuring calibration settings . For labs that prefer digital data management, the osmometer also offers connectivity and software control: it has an RS-232 interface and can be operated through the EuroOsmo 7400 software . Using the software, researchers can control the instrument from a PC, view real-time temperature curves for each measurement (visualizing the freezing point plateau), and save results automatically. The data can be exported in various formats (Excel spreadsheets, text files, etc.) for archiving or integration into Laboratory Information Management Systems (LIMS) . This streamlines record-keeping and facilitates compliance with data integrity standards. The software even supports barcode scanner input for sample IDs , minimizing human errors in sample identification – a small but valuable feature in high-throughput environments.
In terms of applications, the K-7400S is truly multipurpose. It has been successfully used for rapid quality control of isotonic beverages, where its quick 2-minute test cycle and high accuracy are ideal for checking if sports drinks or soft drinks meet their osmolarity targets . In pharmaceutical QC, the K-7400S is used to verify the osmolality of infusion fluids, injectable drugs, and liquid formulations, ensuring each product batch is within the safe osmotic range. The instrument’s compliance with the European Pharmacopoeia osmometry method (Ph. Eur. 2.2.35) means that results from the K-7400S meet stringent regulatory standards . This compliance is a significant advantage for regulated industries – laboratories can confidently use the K-7400S for official quality control tests and in documentation for regulatory agencies. Environmental and research labs also benefit from its precision: whether measuring the osmolality of a tiny biological sample (like a tear fluid in ophthalmic research) or analyzing environmental water samples, the K-7400S provides dependable readings that scientists can trust. With its small footprint and robust build, it saves valuable bench space and stands up to daily use in busy labs . All these features and capabilities make the KNAUER K-7400S osmometer a versatile instrument that enhances the ease and accuracy of osmolarity measurement across diverse scientific fields.
Impact on Industry Standards and Regulatory Compliance
The ability to measure osmolarity with precision has a direct impact on maintaining industry standards and meeting regulatory compliance in pharmaceuticals, food & beverage, and environmental management. In the pharmaceutical sector, pharmacopeial standards (such as those in the European Pharmacopoeia and U.S. Pharmacopeia) often specify osmolality testing for parenteral solutions, dialysis fluids, and ophthalmic products to ensure they are within safe osmotic limits. A device like the K-7400S, which complies with Pharm. Eur. methods , allows labs to perform these tests with confidence that the results will be accepted by regulatory authorities. Precise osmolarity measurements ensure that intravenous infusions are isotonic, preventing potentially dangerous shifts in patient blood chemistry. For example, a standard 0.9% saline IV solution should measure roughly 308 mOsm/L; continuous monitoring and QC of such solutions guard against errors in manufacturing that could alter this value. Regulatory guidelines implicitly demand this level of control – a batch that is out of spec in osmolarity could be flagged as adulterated or unfit for use. By using accurate osmometers, companies not only protect patients but also document compliance with Good Manufacturing Practice (GMP) requirements for product testing.
In food and beverage production, meeting label claims and legal standards is equally important. If a sports drink is sold as “isotonic,” authorities like EFSA (in Europe) require evidence that its osmolality indeed falls in the 270–330 mOsm/kg range . Precise osmolarity measurement provides that evidence. Companies that invest in robust osmometry can consistently validate their formulation and avoid regulatory issues or consumer distrust. It also enables them to fine-tune recipes to meet nutritional standards – for instance, adjusting sugar or electrolyte content to reach the desired osmotic balance. In the realm of environmental regulation, accurate osmolarity (or salinity) data can be part of compliance monitoring for water treatment and discharge. Environmental agencies may set limits on total dissolved solids or salinity for wastewater being released into rivers to prevent ecological harm. Osmolarity measurements (often in conjunction with conductivity readings) help ensure that water treatment processes are effective and that discharges stay within permitted osmotic concentrations. Precise measurement thus underpins adherence to environmental standards designed to protect ecosystems.
Another aspect of compliance is documentation and traceability. Modern osmometers like the K-7400S with its control software EuroOsmo 7400 facilitate this by logging data and allowing electronic records. This is important for audits and reviews – having a clear record of osmolality for each batch of product or each sample tested can demonstrate due diligence and consistent quality control. It helps in standardizing quality across batches and sites, since instruments can be calibrated to international reference standards. The net impact is that industries can achieve higher consistency and reliability in their products. Medications will have the intended tonicity, beverages will deliver on their health promises, and water sources will be monitored for changes in solute content – all thanks to accurate osmolality measurements. In essence, precise osmometry is not just a laboratory procedure; it is a foundation for meeting the stringent standards set by regulators and for protecting public health and safety.
Conclusion
Osmolarity may once have been just a textbook concept, but today it is a practical metric that touches many aspects of our lives – from the IV drip in a hospital to the sports drink in your gym bag. Measuring osmolarity with accuracy and confidence is essential for scientific progress, product quality, and regulatory compliance. Industries such as pharmaceuticals, food & beverage, and environmental science rely on osmometry to ensure that their solutions and products behave as intended and meet defined standards. The freezing point depression method has emerged as a gold standard for these measurements, offering a reliable way to translate physical chemistry principles into actionable quality metrics. Instruments like the KNAUER Osmometer K-7400S exemplify how modern technology can make this process fast, simple, and exceedingly precise. By combining small sample requirements, quick analysis, and robust design with digital intelligence and compliance with pharmacopeial methods, the K-7400S enables laboratories to integrate osmolarity testing seamlessly into their workflows.
In a world where precision in formulation and consistency in production are paramount, osmolarity measurement stands out as a key quality attribute. Whether one is formulating a life-saving infusion, brewing the next sports drink, or monitoring a freshwater supply, understanding and controlling osmolarity leads to better outcomes. As we have seen, the applications of osmometry are diverse, but the core principle remains the same – leveraging the science of osmosis to inform and improve real-world solutions. With advanced tools at our disposal and a clear appreciation of osmolarity’s importance, scientists and industry professionals can ensure that their products not only perform optimally but also adhere to the highest standards of safety and efficacy. In summary, mastering osmolarity measurement is a smart investment into the quality and trustworthiness of scientific and industrial endeavors, and devices like KNAUER’s K-7400S osmometer are invaluable partners in achieving that mastery.
Sources:
1. KNAUER Blog – “Dilute to get more” (Short Bowel Syndrome and Osmometry) – discussion of osmolality in beverages and isotonic solutions.
2. GMI Inc. – Osmometry & Isotonic Drink Analysis – explains the use of osmometry in validating isotonic beverages and introduces KNAUER K-7400S features.
3. MeasurLabs – Osmolality and Osmolarity Testing – definition of osmolarity/osmolality and their use in pharmaceutical and food industry quality control.
4. Wikipedia – Freezing Point Depression Osmometer – principle of freezing point osmometry and its usage in medical and pharmaceutical fields.
5. StatPearls (NCBI) – Osmometer – details on osmometry methods and the 1.86 °C per osmole freezing point depression constant.
6. KNAUER Product Brochure – Freezing Point Osmometry (K-7400S) – technical details of the K-7400S osmometer (sample volume, range, precision) and compliance information.
7. KNAUER Store – K-7400S Semi-Micro Osmometer – product description highlighting instrument design, software, and European Pharmacopoeia compliance.
8. GMI Inc. – Product Description: KNAUER K-7400S Osmometer – features of the K-7400S including two-point calibration, Peltier cooling, and data export capabilities.
9. KNAUER Blog – “Make Your Favorite Drink Isotonic” (MyFDI project) – real-world application of K-7400S in measuring popular beverages, with an example reading of 299 mOsm/kg.
10. Wikipedia – Osmolality in Clinical Chemistry – notes the use of osmometry for urine and plasma analysis and alternative osmometry methods .
For further information on this topic, please contact our author author@knauer.net

